Stefanie Ranf-Zipproth
PER 04 - 0.113
+41 26 300 8817
E-mail
There is a growing global need for sustainable and environmentally friendly strategies to protect crops from pathogens to ensure future food supply and quality. One strategy is to strengthen the natural defense repertoire of plants to increase their resistance to disease.
The focus of our laboratory is on understanding the interactions between plants and bacteria at the molecular level. In an interdisciplinary approach, we study how plants can recognize microbial infections, but also the bacterial virulence strategies.
Plants are permanently exposed to diverse classes of potentially pathogenic microbes. Despite, plants are mostly healthy as they have evolved efficient surveillance and defence systems to ward off microbial invaders. Pathogens, in turn, evolved virulence strategies to evade recognition or suppress defence responses, while plants evolve new recognition strategies. Hence, an evolutionary arms-race exists between pathogens and their potential host plants. We want to understand the molecular mechanisms of plant immune sensing and immune responses as well as microbial adaptation and virulence strategies to support plant breeding and biotechnology in keeping pace with the adaptation of pathogens and in strengthening plant health.
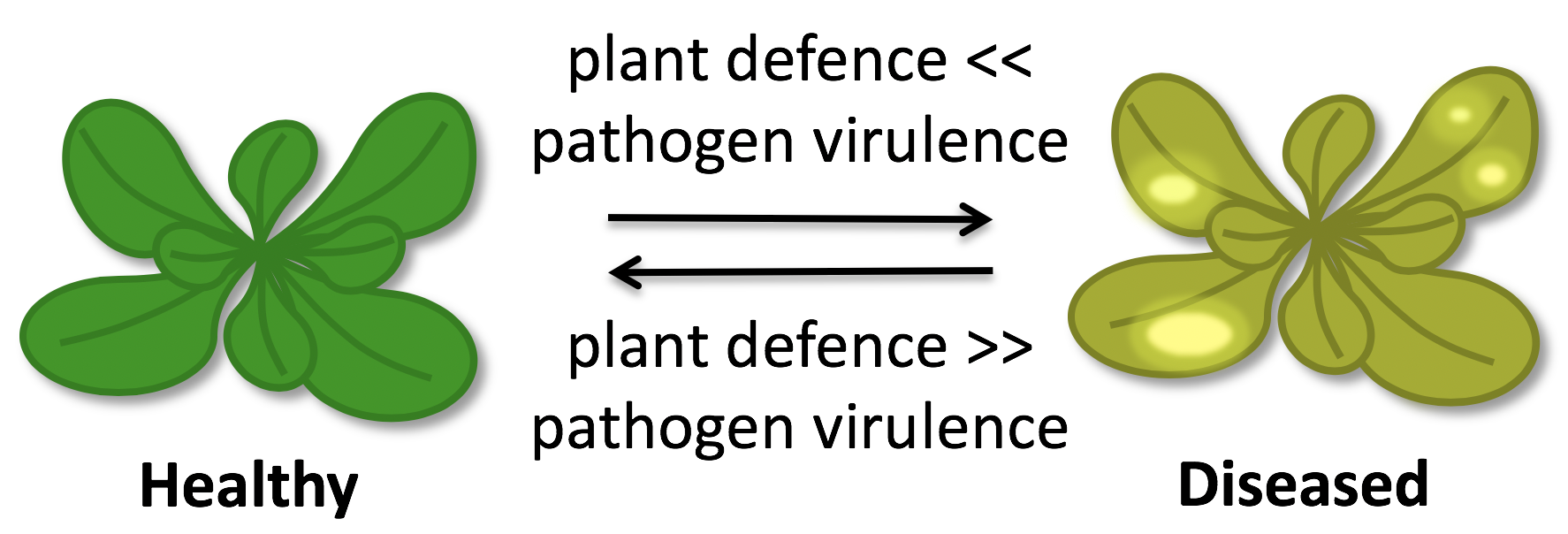
Conserved microbial signatures, so-called microbe-associated molecular patterns (MAMPs), betray the pathogen’s presence to the host. MAMP detection by specific host pattern-recognition receptors (PRRs) is an integral part of the immune system of animals and plants. Cell surface components such as bacterial lipopolysaccharide (LPS), peptidoglycan and flagellin are typical MAMPs as they are vital for microbial survival and common to whole microbial classes. In mammals, MAMPs are sensed by different classes of PRRs e.g. the Toll-like receptors (TLRs), and trigger inflammatory responses. Vertebrates additionally evolved an adaptive immune system employing highly specific antibodies. Plants do not possess specialized roaming immune cells like vertebrates and rely solely on genetically determined innate immune responses that can be executed by almost all plant cells. In plants, sensing of MAMPs by PRRs induces a common set of signalling and defence responses known as pattern-triggered immunity (PTI). Despite some conceptual similarities, MAMP sensing apparently evolved independently in animals and plants.
Our group studies the interaction between plants and Gram-negative bacterial pathogens such as Pseudomonas syringae. We are particularly interested in LPS, a heterogeneous and complex glycolipid macromolecule composed of several domains, that covers ~75% of the Gram-negative bacterial cell surface. LPS is not only vital for survival of bacteria but fulfils multiple roles in interaction with hosts. LPS is sensed in various ways by the mammalian as well as the plant immune system and triggers defence reactions. At the same time, LPS as a highly restrictive barrier, is vital for resistance against antimicrobial compounds released by the host. We want to understand LPS immune sensing and the role of LPS as virulence factor during plant infection on the molecular level by combining expertise in plant biochemistry, microbiology, structural biology as well as analytical chemistry.
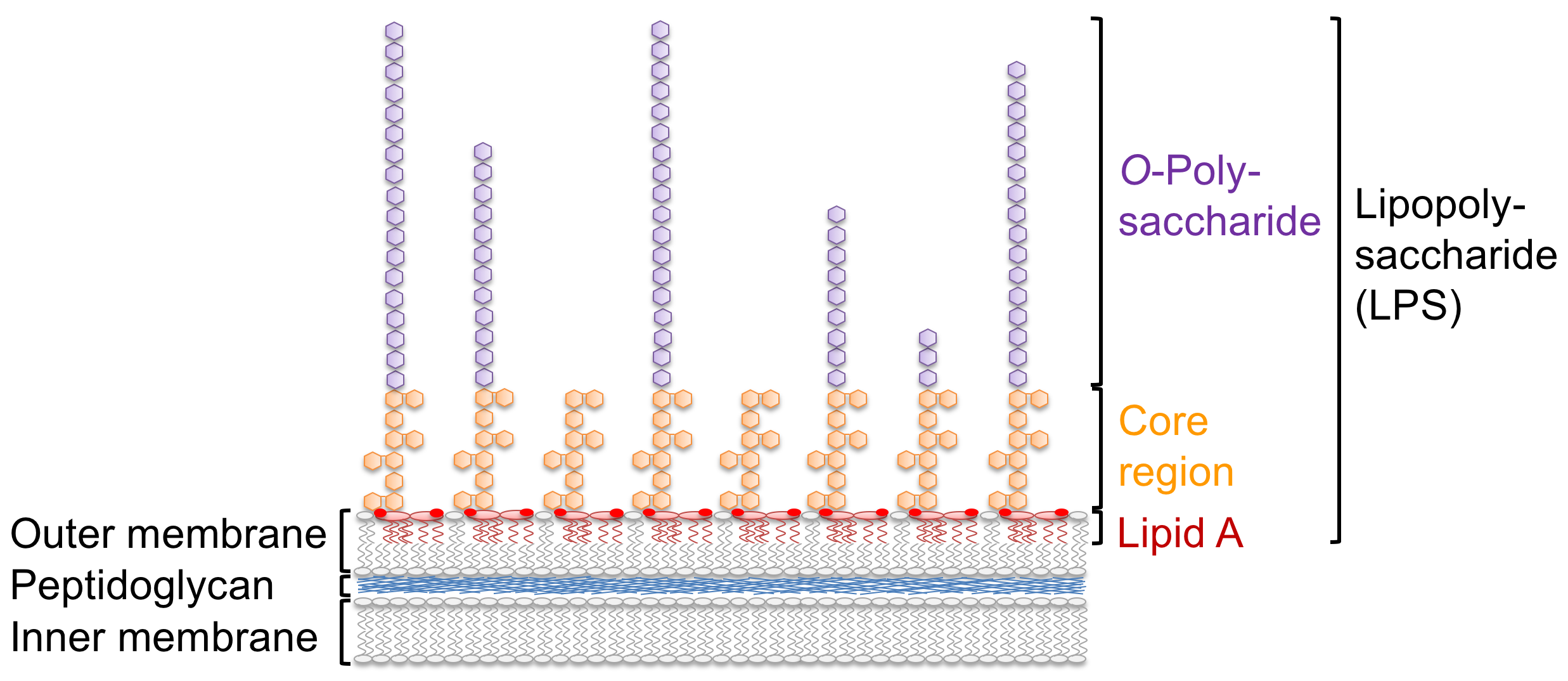
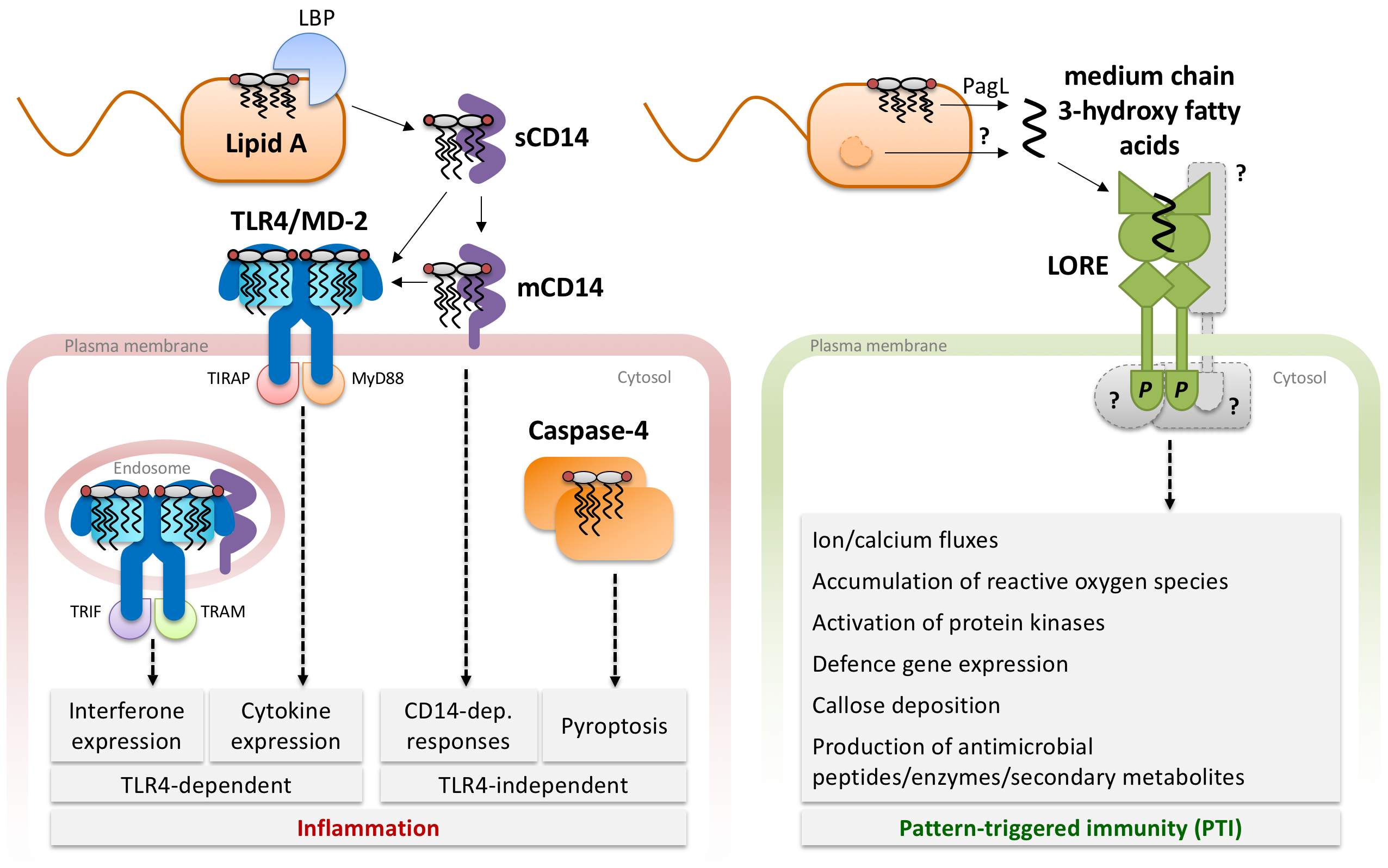
In mammals, LPS is a strong agonist of the innate immune system and its lipid A domain is sensed in trace amounts by extra- and intracellular immune receptors. LPS is also sensed in different plant species, but the perception systems are not yet understood. In a genetic screen for LPS sensing components in the model plant Arabidopsis thaliana, we identified the lectin S-domain receptor kinase LORE (LipoOligosaccharide-specific Reduced Elicitation) (Ranf et al., Nature Immunology, 2015). Interestingly, LORE does not sense LPS itself but free 3-hydroxylated fatty acids of medium chain length, which apparently co-purify with LPS during extraction from bacterial cell material. In Pseudomonas, medium chain 3-hydroxy fatty acids are part of the lipid A moiety of LPS and released during synthesis of penta-acylated LPS in the outer membrane by the lipid A O-deacylase PagL. 3-hydroxy fatty acids are also building blocks of other bacterial compounds and are presumably produced through different metabolic pathways (Kutschera et al., Science, 2019). Hence, in contrast to mammals which sense complex structures such as the lipid A domain of LPS, Arabidopsis plants sense small, low-complexity 3-hydroxy fatty acid metabolites.
Copyright of photos : Astrid Eckert, Technical University of Munich
PER 04 - 0.113
+41 26 300 8817
E-mail