Curzio Rüegg
Professeur-e émérite
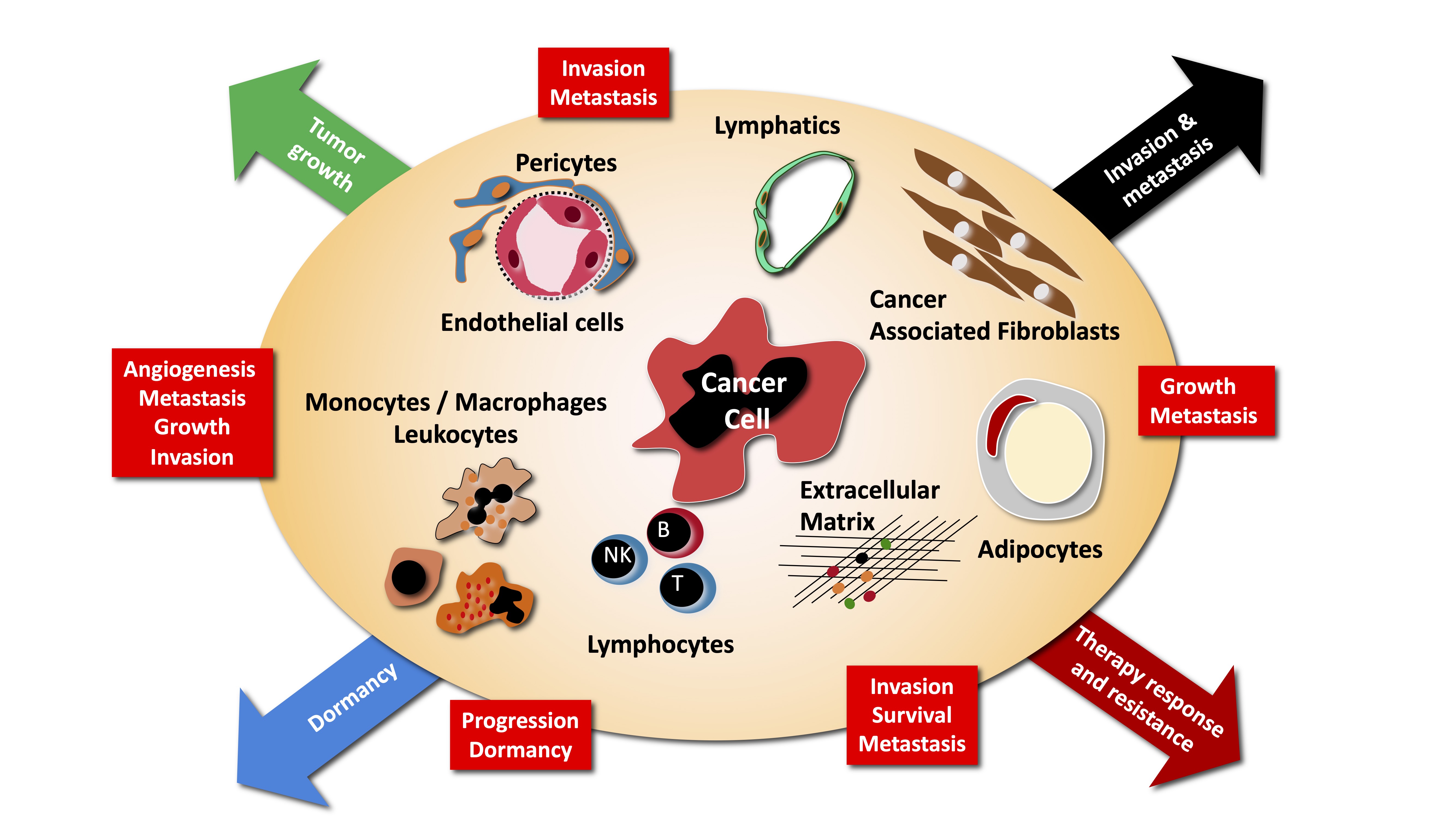
The main research interest of the laboratory is the study of tumor-host interactions. There is growing evidence that the normal tissue co-opted and modified by the growing tumor, provide essential cues to tumor maintenance, dormancy, growth, invasion and metastasis.
We are particularly interested in understanding how the growing tumor modify normal tissue to its advantage, how this modify tissue contribute to tumorigenesis and how therapeutic interventions modify this cross-talk, and what are the consequences. More specifically we are investigating the following aspects of tumor - host interaction:
Cliquer ici pour la version en Français
DNA is the molecule that naturally carries genetic information in our cells. However, DNA molecules can also be employed as building material to engineer predefined structures with programmable functions at the nanoscale scale. The employed technique is called DNA origami, inspired by the Japanese art of paper folding to create three-dimensional objects. In the context of the NCCR program in bioinspired materials we are developing DNA nanosensors for specific and sensitive detections of tumor-derived biomarkers.
A first examples is a book-shaped DNA origami biosensor that produces a strong fluorescent optical readout upon binding of small RNAs (miRNA) specific for breast cancer. Simultaneous detection of two miRNA can be achieved within ten minutes with a very low limit of detection (low picomolar range). A second is a DNA sensor based on position-dependent signal recognition using the super-resolution microscopy technique called DNA-PAINT (Point Accumulation for Imaging in Nanoscale Topography), with extremely high sensitivity (low femtomolar range) and single-base specificity for miRNA. We are currently developing approaches to detect mRNAs at high specificity ad sensitivity. The long-term goal of these projects is to develope rapid, safe, simple, and low-cost clinically applicable tests for cancer detection and monitoring to improve cancer diagnostic and patient care.
An additional important research topic we are pursuing in the laboratory is focused on fibrosis, particular lung fibrosis. Lung fibrosis can arise from various origins (systemic sclerosis or direct damage to the lungs). The involvement of epithelial cells, blood vessel endothelial cells, immune and inflammatory cells, and fibroblasts are key elements of the disease progression. Typically, tissue damage and inflammation/immune dysregulation are followed by an extracellular matrix deposition by the myofibroblasts. We are particularly interested in better understanding the role of endothelial cells in the first phase of systemic sclerosis and associated lung damage development and how to characterize novel therapies for targeting myofibroblasts in the established disease. Fibrosis is also relevant to cancer and knowledge form this project will be relevant to address cancer-related fibrosis.
The current paradigm defines metastasis as a process driving the selection of cells with advantageous traits that allow them to overcome the diverse environmental defenses against the ectopic growth of cells in tissues different from the ones of origin. Many genes involved in promoting metastases have been recently uncovered, including matrix metalloproteinases, chemokines and their receptors, integrins and integrin-dependent signaling events. Many questions remain open in this field. Several important questions remain open at this point. A critical one is the timing of metastasis. Do metastatic cells disseminate early, i.e. at a time when primary tumors are not yet detected, or late, i.e. at a time when primary tumors reach a critical mass, during cancer progression ? How do disseminated cells interact with normal tissue at metastatic sites, in particular in the brain? What is the contribution of bone marrow derived cells to metastasis ? We are pursuing some of these questions using various models of cancer metastasis, mostly of breast cancer.
Several projects conducted in the laboratory are based on clinically relevant questions, with the goal to be able to develop new strategies aimed at improving cancer management and patient quality of live, though improved cancer diagnosis, therapy and monitoring. We are collaborating with several local, national and international cancer centers to design, perform and analyze clinical-laboratory (translational) studies aimed at testing and validating hypothesis based on results generated in the laboratory. Currently ongoing studies include:
MAGI1 is a cytoplasmic scaffolding protein stabilizing cadherin-mediated cell–cell adhesion in epithelial and endothelial cells. Clinical-pathological and experimental evidence indicates that MAGI1 expression is decreased in some inflammatory diseases, and also in several cancers, including colo-rectal, and breast cancers. We originally reported that MAGI1 acts as tumor suppressor in colorectal cancer, is upregulated by NSAIDs in cancer cells, by shear stress in endothelial cells and promote NO production, and its loss in ER+ breast cancer promotes cancer progression. We are now unraveling the effects of MAGI1 loss on oncogenic signaling and fundamental cell functions, as well as mechanisms leading to MAGI1 loss in cancer cells.
Contribution of bone marrow-derived cells to tumor progression and metastasis
Cells recruited to the tumor microenvironment, in particular BMDC and inflammatory cells, contribute to tumor progression, by establishing paracrine relationships with the tumor cells. In particular monocytes/macrophages, are important sensors of tissue hypoxia and necrosis. We are characterizing the mechanisms of mobilization of these cells, their contribution to local tumor growth and their role in generating premetastatic niches.
Inflammatory mechanisms of tumor promotion
Inflammation promotes tumor progression through various mechanisms including expression of COX-2 and prostaglandin production. COX-2 acts as tumor promoter by inducing angiogenesis and stimulating tumor cell survival and motility. We are interested in identifying downstream target genes of COX-2 and molecular mediators of its effects on endothelial cells and tumor cells.
Impact of angiogenesis and antiangiogenic therapies to tumor progression
Angiogenesis is thought to promote tumor growth though the delivery of oxygen and nutriments to the growing tumor, but evidence suggests that additional mechanisms may be involved. We are interested in unraveling paracrine effects of angiogenic vessels on primary tumor growth, tumor invasion and metastasis formation. One signaling pathways we are analyzing is the Akt/PKB pathway, which has potent angiogenic activity. Furthermore, while it has been traditionally assumed that antiangiogenic treatments will not face the problems of resistance as observed during chemotherapy, emerging evidence indicates that indeed tumors can escape angiogenic blockade. We are interested in identifying mechanisms of escape and new molecules as candidate therapeutic targets to prevent/treat escape.