24.05.2013
Better ionic conductivity due to the stacking principle
Ions which are vital for life are continually being transported into and out of the cell membranes of all living beings and plants. Researchers in the team of Prof. Katharina Fromm of the Chemistry Department of the University of Fribourg have now developed a material whose components arrange themselves of their own accord into channel structures, thus enabling ion transport.

At the molecular level, the stacking of components enabling ion transport is not a child's game. (Image: Thinkstock)
Have you ever tried stacking ring-shaped objects: Frisbees, for example? What usually happens is that, after reaching a height of a few centimetres, the stack collapses or slips. But if you have a square framework the size of the Frisbees (let’s say a tall box with a hole at the top and bottom – see illustration below), you can use it to stack as many such objects as you like without them slipping. The centre of the stack of Frisbee discs forms a channel into which you can then insert other objects, for example: balls. The balls can move up and down within this channel or simply move through the channel if the box containing the discs is raised.
The Fromm research team have succeeded in imitating such a structure at the molecular level through the self-arrangement of the components involved. Its scaffolding, or framework, is formed by rod-shaped, negatively charged molecules, so-called polyhalides, and within this framework the disc-shaped neutral components (in this case crown ethers) arrange themselves into stacks. Without the framework the crown ethers won’t stack. Into the channels which form within the stack, so-called cations (positively charged ions), such as sodium or potassium ions, for example, can then be inserted. The structures of parallel channels which thus occur can form macroscopic single crystals (see the illustrations of the publication’s appendix). The cations can move through the channels of these crystals like the balls through the stacked Frisbee discs; thus the single crystals are good ionic conductors.
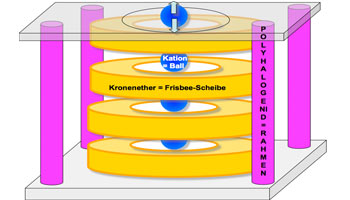
cation = ball / crown ether = Frisbee disc / polyhalide = framework
Such cation-conducting combinations play an important role in Lithium-ion batteries, for example, where lithium ions need to be transported – a process which takes place every day in all mobile phones, laptops or iPads.
A similar process occurs in our body’s cells where ions are constantly entering and leaving. This process is vitally important for our electrolyte balance. The single-crystalline materials described in the team’s published research paper could also be used in microanalysis in cases where it is a matter of ascertaining the presence of extremely small quantities of ions in tiny amounts of fluid.
Link to the publication:
http://onlinelibrary.wiley.com/doi/10.1002/anie.201208195/pdf
Contact details
:Prof. Dr. Katharina M. Fromm, Chemistry Department, Fribourg Center of Nano-Materials FriMat, University of Fribourg, 026 300 87 32, katharina.fromm@unifr.ch, http://www.chem.unifr.ch/kf/index.html
